Products
winTop therapy® Cell Cryopreservation Bag (50mL)
Product Code: FK-005
Capacity: 50mL
Material: Medical-grade EVA
Freeze/Fill volume: 10-30mL
Packaging: Sterile individual packaging, 50 units/box
List Price: Price inquiry
Product Description
Cell therapy has been widely researched in recent years, especially stem cell and immune cell therapy has a broad prospect, which involves regenerative medicine, clinical application, tumor treatment, chronic disease prevention and treatment, medical cosmetology, slowing down the aging of the body, genetic testing and health intervention and other fields. Our winTop therapy® series of cell freezing bags have been tested to meet the freezing requirements of various biological products. The product is made of international advanced medical biomaterials (EVA/composite EVA), which is safe, reliable and suitable for the frozen storage of biological products at any temperature, and it can withstand ultra-low temperature (-196℃) for a long time without rupture, and maintain good cell activity.
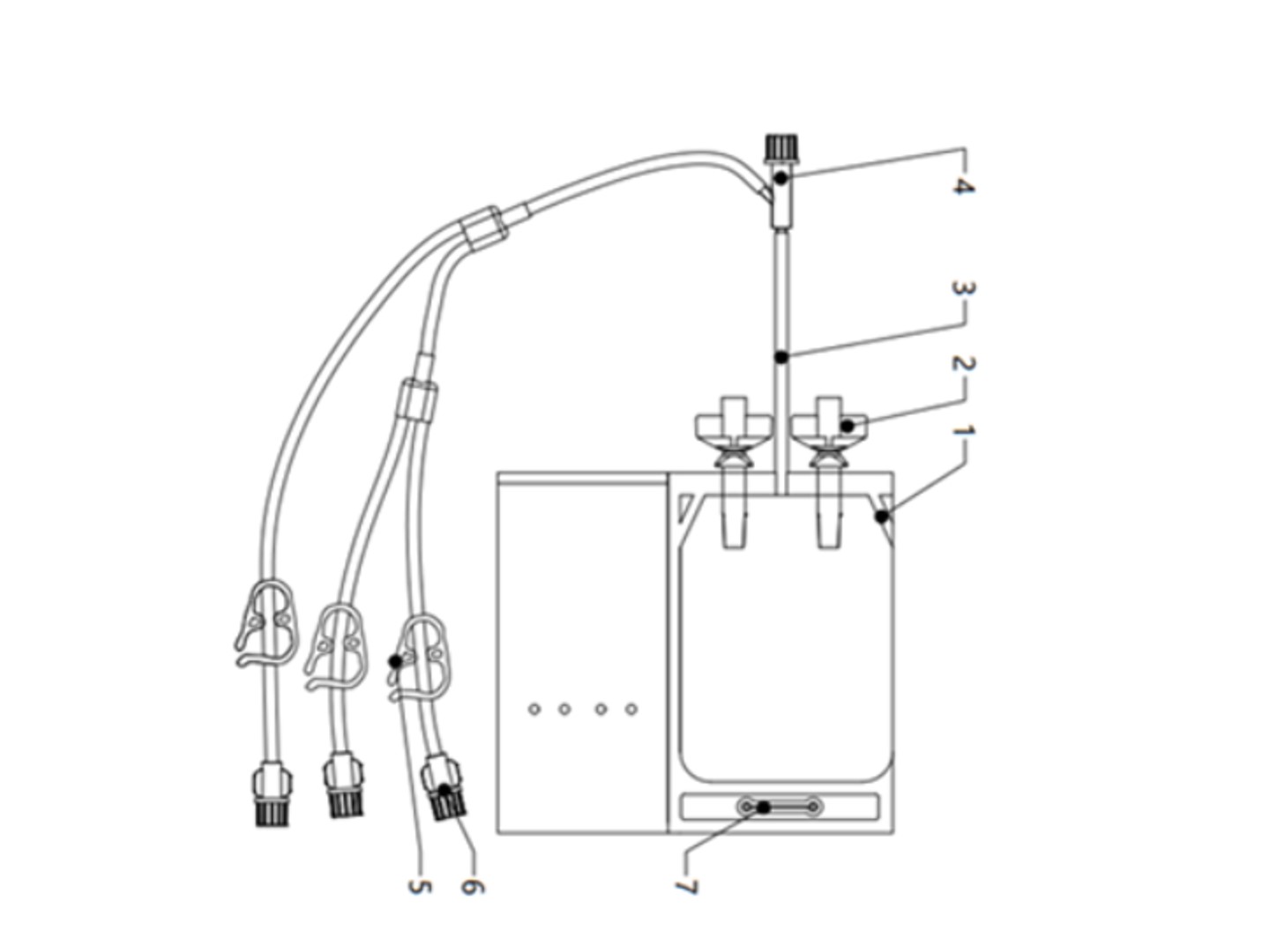
Each set contains the following components: freezing bag + single line + 2 butterfly ports + Y-connector + clip + 2 luer female connectors + 1 luer male connector.
Key Features
Innovative Design:
Provide single or multi-chamber bags to meet diverse cell therapy needs; Special emptying structure reduces waste;Extending the EVA pipeline facilitates quality control sampling.
Safe and reliable:
Individual/sterile packaging and overwrap bags can increase sample safety; GMP-grade manufacturing facility to ensure the quality of products; ethylene oxide EO or irradiated EB sterilization, aseptic, no heat source, single-use.
High standard materials:
EP/USP Standard Medical grade EVA or composite EVA, with good biocompatibility;with long term resistance at -196°C for ultra-low temperature storage.
Product Specifications(Other specifications can be customised)
| Cat No. | Pruduct name | Specifications | Suggested Liquid Volume |
Packaging Specifications |
| FK-008 | winTop therapy®cell preservation bag 15 mL | 15 mL | 3-5 mL | Individual packaging,50 sets/box |
| FK-025 | winTop therapy®cell preservation bag 15 mL(excluding label area) | 15 mL | 3-5 mL | Individual packaging,50 sets/box |
| FK-010 | winTop therapy®cell preservation bag 25 mL | 25 mL | 10-20 mL | Individual packaging,50 sets/box |
| FK-005 | winTop therapy®cell preservation bag 50 mL | 50 mL | 10-30 mL | Individual packaging,50 sets/box |
| FK-007 | winTop therapy®cell preservation bag 50 mL(excluding label area) | 50 mL | 10-30 mL | Individual packaging,50 sets/box |
| FK-016 | winTop therapy®cell preservation bag 250 mL | 250 mL | 30-70 mL | Individual packaging,50 sets/box |
Applications
- Biopharma R&D: Drug screening (tumor cells, gene-edited lines), viral vector production (vaccines).
- Clinical Therapy: CAR-T/NK cell preservation, hematopoietic stem cell transplantation, reproductive medicine.
- Regenerative Medicine: Stem cell banking (MSCs, iPSCs), 3D bioprinting.
- Biobanking: Tumor organoids, rare disease cell lines, umbilical cord blood storage.
- Agriculture & Industry: Genetic resource preservation, industrial enzyme/microbe storage.
Biosafety Validation
| Test Item | Result |
|---|---|
| Factory Inspection Report | √ |
| EVA Material Biocompatibility | √ |
| Bacterial Endotoxin & Sterility Test | √ |
| Acute Toxicity/Hemolysis/Cytotoxicity Test | √ |
| Non-Soluble Particle Test | √ |
For custom orders, technical support, or bulk pricing, reach out to us now!
Empowering Innovation in Cell Therapy and Beyond.