In recent years, umbilical cord-derived Mesenchymal Stem Cells (UCMSCs) have attracted much attention in the field of regenerative medicine, especially in the treatment of inflammation-related diseases.
Background of the study
Mesenchymal stem cells (MSCs) have shown great potential in the treatment of a wide range of diseases due to their multidirectional differentiation potential, low immunogenicity, and potent immunomodulatory ability.UCMSCs, an important source of MSCs, have become a hot topic of research due to their abundant source, easy accessibility, and non-invasive collection method. Previous studies have shown that a single infusion of CLV-100 is safe in healthy volunteers and has a dose-dependent immunomodulatory effect. However, these studies have focused primarily on short- to medium-term safety and there is a relative paucity of data on long-term effects.
The aim of this study was to assess the mid- to long-term safety and immunomodulatory effects of a single infusion of CLV-100 in healthy subjects through five years of follow-up, and to further explore its effects on aging and inflammation-associated debility.
Clinical research methods and study design
The research team recruited 11 healthy volunteers between 2017 and 2018, divided into a low-dose group (65 million cells, LD group) and a high-dose group (130 million cells, HD group). All subjects received a single intravenous infusion of CLV-100 and were followed up on days 2, 30, 90, and 180 post-infusion. Follow-up was interrupted from 2019 to 2022 due to the COVID-19 outbreak, but all subjects remained in contact with the clinic.In March and April 2023, all subjects were invited to participate in a five-year follow-up to assess the long-term safety and immunomodulatory effects of CLV-100 infusion.
Assessment of indicators
Primary assessment metrics included safety of clinical and subclinical outcomes with a focus on death, hospitalization, related adverse events, and cancer. Secondary assessment metrics were based on subclinical assessment of inflammatory cytokines in serum.
Data collection and analysis
During follow-up, subjects’ medical histories, blood samples and serum were collected, and several clinical and subclinical assessments including complete blood counts, liver and renal function tests, lipid tests, inflammatory cytokines and growth factors were performed. Data were analyzed using SPSS and Prism software, and the Friedman test, Wilcoxon signed rank test, and Mann-Whitney test were used to compare data at different time points.
Findings of the study
Subject characteristics
All 11 subjects (4 males and 7 females) completed five years of follow-up. The median age of the subjects was 56 years and their BMI was 22.40 kg/m². All subjects remained functionally independent and healthy during the follow-up period.
Security Assessment
During the five-year follow-up period, none of the subjects reported adverse events, major health problems, or hospitalizations related to CLV-100 infusion. Blood test results showed that all subjects had clinical parameters within normal limits, including complete blood counts, lipids, liver and kidney function, and tumor markers. Of particular note, major organ health parameters remained stable despite the fact that some subjects were over 60 years of age.
Immunomodulatory effects
The results of the study showed that the anti-inflammatory effect in subjects after CLV-100 infusion was consistently significant in the HD group. Specifically, levels of the anti-inflammatory cytokine IL-1Ra were significantly higher in the HD group, while levels of the pro-inflammatory cytokines IL-6 and TNF-α were significantly lower. These changes persisted at five-year follow-up, suggesting a long-term immunomodulatory effect of CLV-100. In contrast, these changes were not significant in the LD group.
In addition, the study evaluated levels of the growth factors VEGF, HGF and TGF-β. The results showed that VEGF and HGF levels were significantly lower at the five-year follow-up, while TGF-β levels were significantly higher. These changes were particularly pronounced in the HD group, but not in the LD group.
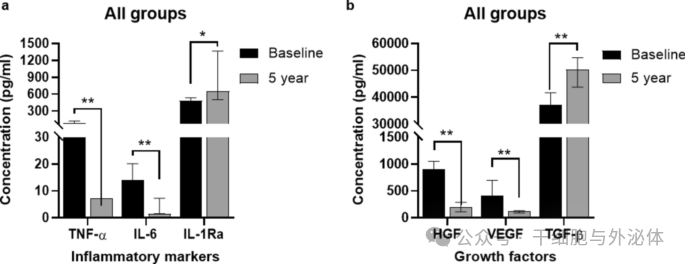
Figure: Comparison of inflammatory marker (a) and growth factor (b) levels in all groups (n = 11) at baseline and during the 5-year follow-up period. The Wilcoxon signed rank test was used to compare the differences between the two time points. *p < 0.05, **p < 0.01
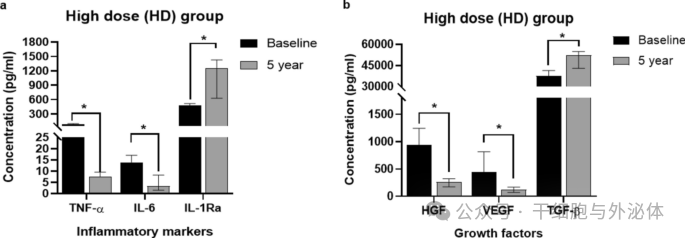
Figure : Comparison of inflammatory markers (a) and growth factors (b) levels in the high-dose (HD; n = 6) group at baseline and during the 5-year follow-up period. The Wilcoxon signed rank test was used to compare the differences between the two time points. *p < 0.05
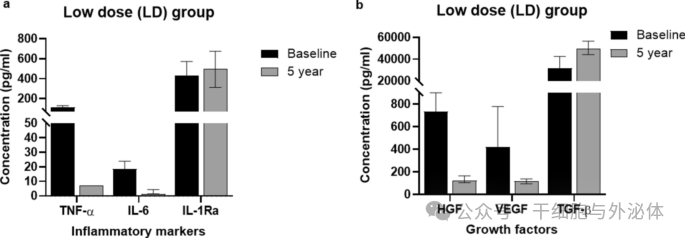
Figure : Levels of inflammatory markers ( a ) and growth factors ( b ) in the low-dose (LD; n = 3 at baseline, n = 5 after 5 years) group compared with baseline and 5-year follow-up data. The Wilcoxon signed rank test was used to compare differences between the two time points.
Impact of Age on Effectiveness
The study further analyzed the effect of age on the effectiveness of CLV-100 infusion. Subjects were categorized into younger (<60 years) and older (≥60 years) groups, and the results showed no significant differences in the levels of inflammatory cytokines and growth factors between the two groups. This suggests that CLV-100 infusion has the same potential protective effect in older subjects.
Deliberations
Safety of CLV-100 Infusion
This study demonstrated that a single infusion of CLV-100 exhibited a favorable medium- to long-term safety profile in healthy subjects. All subjects experienced no serious adverse events and clinical parameters remained stable during the five-year follow-up period. These results are consistent with the results of previous studies on the safety of UCMSCs in diseased patients, further confirming the safety of UCMSCs in clinical applications.
Immunomodulatory effects of CLV-100
The results of the study showed that the anti-inflammatory effect of CLV-100 infusion in the subjects continued to be significant in the HD group, suggesting that CLV-100 has long-term immunomodulatory capacity. The elevation of the anti-inflammatory cytokine IL-1Ra and the reduction of the pro-inflammatory cytokines IL-6 and TNF-α may be related to the interaction of CLV-100 with immune cells through paracrine effects. These changes contribute to a reduction in the systemic inflammatory response, which may reduce the risk of inflammation-related diseases.
Effects of CLV-100 on Aging and Frailty
The present study also found that CLV-100 infusion may have a potential protective effect against aging and inflammation-related debility. Despite the fact that some subjects were over 60 years old, their major organ health parameters remained stable and their inflammatory cytokine levels were significantly reduced. This suggests that CLV-100 may help to slow down the aging process and reduce the occurrence of inflammation-related debility. However, due to the lack of a control group in this study, whether these effects are entirely attributable to CLV-100 infusion still requires further study.
Research limitations
Despite the important results of this study, there are some limitations. First, due to the limited serum sample size, the study used a multiple array kit rather than an ELISA kit for cytokine and growth factor quantification, which may have affected the accuracy of the results. Second, this study lacked a control group to directly compare the effects of CLV-100 infusion with placebo. Finally, the follow-up from 2019 to 2022 was interrupted due to the COVID-19 outbreak, resulting in missing data.
Conclusions
This study evaluated the medium- to long-term safety and immunomodulatory effects of a single infusion of CLV-100 in healthy subjects through five years of follow-up. The results showed that CLV-100 infusion demonstrated a favorable safety profile and long-term immunomodulatory capacity in healthy subjects. In addition, CLV-100 may have potential protective effects against aging and inflammation-related debility. Larger randomized controlled trials are needed in the future to further validate these findings and to explore additional possibilities for CLV-100 in clinical applications.
Disclaimer: The information in this article comes from the Internet, this article is only for the purpose of knowledge exchange and sharing and popularization of science, does not involve commercial propaganda, and does not serve as relevant medical guidance or medication advice. Please contact us for deletion if there is any infringement. Source: Stem Cells and Exosomes


